A New Normal in Pharmaceutical Waste Management

Bring your pharmaceutical waste management into the modern era – by developing a hazardous vs non-hazardous pharmaceuticals segregation program
Pharmaceutical waste management is one of the most misappropriated waste streams generated out of a hospital, pharmacy or healthcare environment. Its differing tiers of risk association and the complexity of navigating these tiers renders users to either be extra diligent in waste categorization and secure pharmaceutical disposal, or woefully complacent.
In direct proportion to the risk it poses to humans and the environment, it is time to introduce a New Normal in Pharmaceutical Waste Management. As Arthur Burt once said “Nothing happens until the pain of remaining the same outweighs the pain of change.”
Explore ways to normalize your pharmaceutical waste program’s costs, risk and environmental impact. Feel free to jump ahead by clicking one of the topics below:
- Pharmaceutical Generator Status, how to impact your classification
- Pharmaceutical Waste Containment; putting safety and security first
- Eliminating the risk and environmental burden of disposable containers
- Overclassification and Formulary; eliminating risks, environmental and cost impact
- Lowering costs by upwards of 50% through non-haz pharma segregation
1. Generator Status, how segregation can impact your facility’s hazardous waste volumes
Let’s start with what Generator Status means for your facility, and why it is in a healthcare facility’s best interest to correctly identify their hazardous waste volumes. The EPA categorizes generators of hazardous waste into 3 distinct classes, VSQG (Very small quantity generators, also known as CESQG – conditionally exempt), SQG (small quantity generators), and LQG (large quantity generators). Each status is directly related to the volume of hazardous waste that a healthcare generates and imposes specific storage and waste accumulation time requirements. For an LQG, waste accumulation is capped at a maximum of 90 days on site and a biennial hazardous waste report must be submitted every two years, but the reality is that many hospitals can avoid these higher restrictions because they shouldn’t generate this much hazardous waste volume.
Daniels recently worked with Iredell Memorial Hospital, a 247 bed facility in North Carolina to combat the challenges they were having with segregation of pharmaceutical waste. Classified as an LQG hazardous waste generator, they were generating 4 times the national average of hazardous waste for like-size hospitals due to non-hazardous waste misappropriation.
Working in partnership with Iredell, we facilitated a full overhaul of their pharmaceutical waste disposal program:
- Ran a formulary analysis to determine what hazardous pharmaceuticals were being used in the facility
- Conducted “black bin” audits to identify over-classified pharmaceuticals in the hazardous waste stream
- Mapped all hospital locations generating non-hazardous pharmaceuticals
- Implemented Daniels Pharmasmart system for the separation of non-hazardous pharmaceuticals
- Downsized all 17gal black bins to 3gal black hazardous containers
- Facilitated staff education and developed custom waste segregation tools
- Used waste audits to identify training gaps and introduce corrective measures
Since implementation, Iredell Memorial Hospital saw its status reduced from an LQG generator to an SQG generator of hazardous waste volumes, together with achieving a 35% cost reduction. Mis-categorized non-hazardous pharmaceuticals can have a significant impact on a hospital’s bottom line! Whether a hospital or outpatient healthcare facility, you can directly influence your hazardous waste spend through the implementation of an effective pharmaceutical segregation program.
The Daniels Team provided subject matter experts which immediately developed credibility with Department Directors to help break down silos and hardwire the new waste stream. I believe we could not have obtained these types of results if it were not for Daniels.
Larry Pizzorni
2. What does a safe pharmaceutical waste container look like?
Working within hospital environments for more than 40 years across six countries, we have seen pharmaceutical waste put under increased scrutiny and federal regulation due to the proliferation of opioid abuse globally. Beyond the fines and legal implications of non-compliance, there lies something much more important – human life, and therein a responsibility on healthcare institutions to provide a safe risk-free environment for clinical staff. 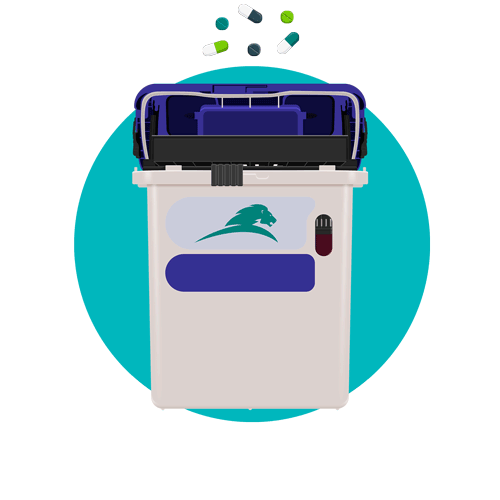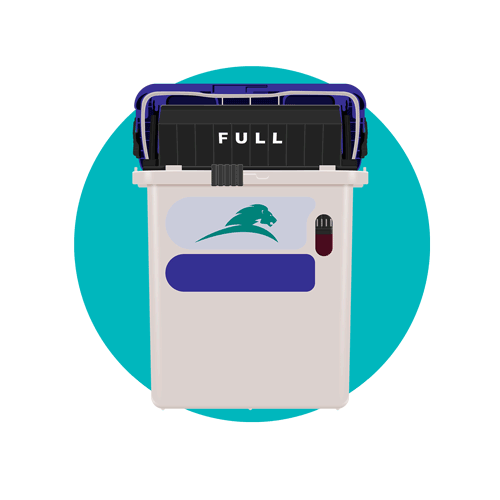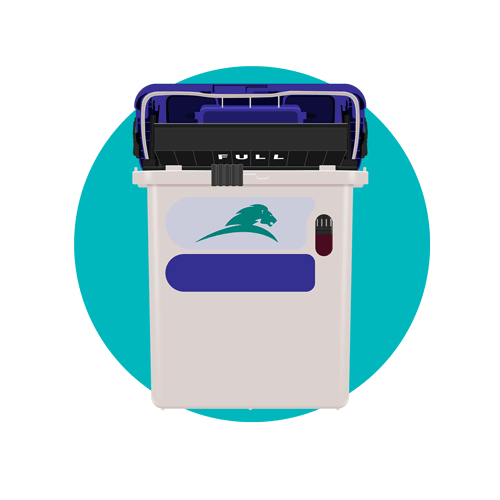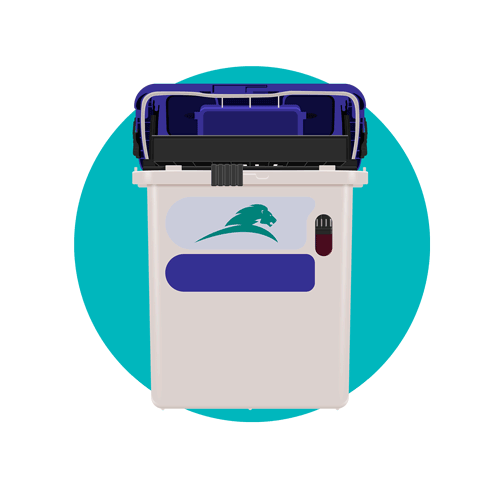
What are the important safety and security features of a pharmaceutical waste container?
- Inbuilt tamper-proof locks which can be secured once the container is full
- Locking brackets that secure a container onto a wall, preventing unauthorized removal
- Restricted container opening that prevents access to contents
- Inbuilt overfill mechanism to prevent overflowing of contents
- Single piece design, no detachable lids or components that could be disassembled
- Mobile ability to be safely wheeled as close as possible to the point of waste generation
- Hands-free disposal design, eliminates risk of needlestick injury or potential infection transfer risk
Daniels Pharmasmart not only meets, but exceeds all of these safety and security requirements. As a DOT approved container for the containment of non-hazardous pharmaceuticals, it can be transported without assembly or secondary packaging and is the only container in the US without a single recorded incidence of sharps penetration. Furthermore, Daniels’ universal bracketing system enables the Pharmasmart to be used with sharps, medical waste and trace chemotherapy waste containers for on the spot segregation as an integrated mobile solution.
3. Eliminating the costs, safety risks, and environmental burden of disposable containers
Let’s cut straight to the point… when is it ever beneficial from a cost or environmental standpoint to needlessly throw away plastic? The dramatic increase in disposable plastic products produced over the last several decades has been extraordinary. Of the 300 million tons of plastic manufactured every year, only 50% of it is for single-use purposes; where reusable products are an option, we should exercise environmental stewardship.
In the healthcare industry, 33% of plastic generated from healthcare waste streams is the plastic container itself… i.e. you’re not only paying more for a disposable container, but you’re also paying to dispose of overclassified plastic! Switching to a reusable non-hazardous waste container will drive both safety and environmental outcomes:
- Eliminate the purchase and disposal cost of single-use containers for non-hazardous waste
- Protect staff with a container designed with safety features comparable to the risk profile of pharmaceuticals
- For every 1 Daniels Pharmasmart container you use, you save 500+ containers from being manufactured and landfilled
 4. When to use a formulary analysis and the benefits of correct pharmaceutical waste classification
4. When to use a formulary analysis and the benefits of correct pharmaceutical waste classification
Approximately 5% of the average drug inventory in a hospital is deemed hazardous waste by the EPA, the problem is that without identifying “which drugs” fall into this 5%, a significant volume of non-hazardous pharmaceuticals are overcategorized into the hazardous waste stream, incurring at minimum a 50% increase in waste disposal spend. It is our recommendation that all hospitals conduct a formulary analysis of their drug inventory once a year and update waste segregation and waste disposal training accordingly to ensure clinical staff are competent in hazardous vs non-hazardous drug classification.
Incorrect pharmaceutical waste classification can have the following impact:
- Inflated costs by overcategorizing non-hazardous pharmaceuticals into black bins – the highest cost category
- Risk of fines and compliance infringements for under-classified hazardous waste being disposed into non-hazardous waste streams such as RMW, trace chemo or non-haz pharma
- Environmental risk of hazardous drugs being treated incorrectly
- Pharmaceuticals exposed to unauthorized access and misuse
- Incorrect hazardous waste generator status applied to the facility
5. Lower your facility’s pharmaceutical waste disposal costs by upwards of 50%
Implementing an effective pharmaceutical waste program requires a holistic look at everything that impacts waste identification, disposal and movement – and that requires a look at not only the systems put in 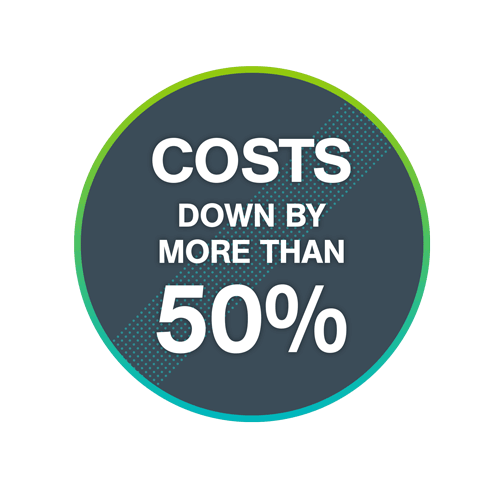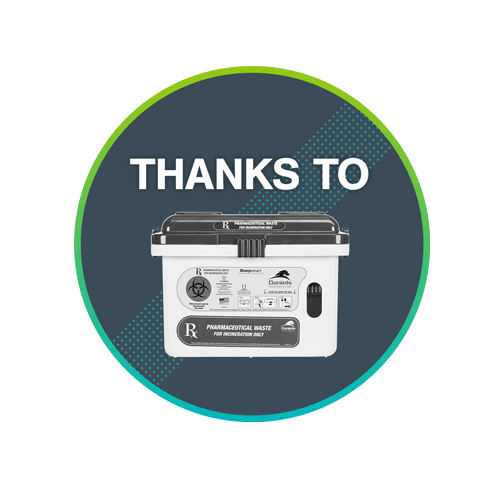 place, but also the humans!!! And when humans are involved, unfortunately there’s no “magic pill” solution for getting results, just a very concerted and focused plan on developing a right-fit program that is simple for clinicians and clinical staff to adopt.
place, but also the humans!!! And when humans are involved, unfortunately there’s no “magic pill” solution for getting results, just a very concerted and focused plan on developing a right-fit program that is simple for clinicians and clinical staff to adopt.
Like many hospitals we have worked with, Mission Hospital had no clear waste segregation plan for its pharmaceutical waste management. Black bins were installed all over the hospital making it a default option for discarding of not only hazardous waste but also RMW, non-hazardous pharmaceuticals and general trash. Mission Health had a fulltime EVS staff member responsible for moving black bins from the hospital wards to the dock and was paying for the disposal of between 36-45 x 55gallon boxes each week.
We won’t go through the details of what we did… you can find out more here. But suffice to say, after our team implemented a full segregation program, Mission’s hazardous waste volume went down to 6 x 55gallon boxes each week and reduced their waste disposal spend by more than 50%! If you’re curious…

The future of pharmaceutical waste management is here
The opioid crisis in America has revealed the fragility of our human environment and exposed the need to remove the ability for misuse. A New Normal in pharmaceutical waste management requires a higher standard of safety and security in waste containment, and a dedication to environmental stewardship that protects our planet for years to come. Talk to us about how Daniels Health can partnership with your organization in implementing an effective pharmaceutical waste program.
Let's Talk!
Your time is valuable, and we don’t want to play hard to get. You can either phone us directly on the details listed on our contact page, or feel free to fill out this short form and one of our team members will get back to you as quickly as possible.
