How to Handle Regulated Medical Waste from Mpox Cases
On May 20, 2022, the Centers for Disease Control and Prevention (CDC) issued a health advisory titled Monkeypox Virus Infection in the United States and Other Non-endemic Countries – 2022. This notification serves to provide hospitals and other healthcare facilities with information on the handling of regulated medical waste (RMW) generated from the care of suspected or confirmed monkeypox (Mpox) patients. The following is a general guideline, so please make sure to follow all applicable local, state, and federal requirements that apply to the transport, treatment, and disposal of RMW and infectious substances.
TOPICS WE WILL COVER:
1 / Monkeypox Virus Classification
2 / Case Confirmation and Clade Identitifcation
3 / Handling, Packaging, and Transport of Monkeypox Waste
5 / Any Clade Except West African
Monkeypox Virus Classification
Previous studies have defined two distinct Monkeypox clades, the West African and Central African (Congo Basin) clade, which differ in how the disease presents in people, the severity of its effects, and how readily it spreads. The West African infection is less severe and associated with less human-to-human transmission compared to the Congo Basin clade. The June 2022 US National Security Council (NSC) Guide states the management of wastes generated from patients infected with monkeypox is determined by which clade has been identified.
Case Confirmation and Clade Identification
The CDC Monkeypox website advises that your local state health department be notified of a suspected case before lesion material is collected. Laboratory Response Network laboratories provide orthopoxvirus testing on lesion specimens, and CDC Poxvirus Lab performs monkeypox clade identification. The CDC states the formal test report is available in five to seven days; however, the Poxvirus Lab endeavors to relay the results to the sender within a day of receiving the specimen.
Handling, packaging, and transport of monkeypox wastes
CDC advise staff to wear appropriate PPE as recommended by the CDC when handling monkeypox wastes. In reference to the June 2022 NSC Guide, waste contaminated with or suspected of containing monkeypox virus is managed as follows:
- West African clade is managed as routine RMW (UN 3291)
- Congo basin clade is managed as Category A Infectious Substance (UN 2814).
The following table from the 2022 guide outlines the procedures for handling, packaging, and transporting both clades.
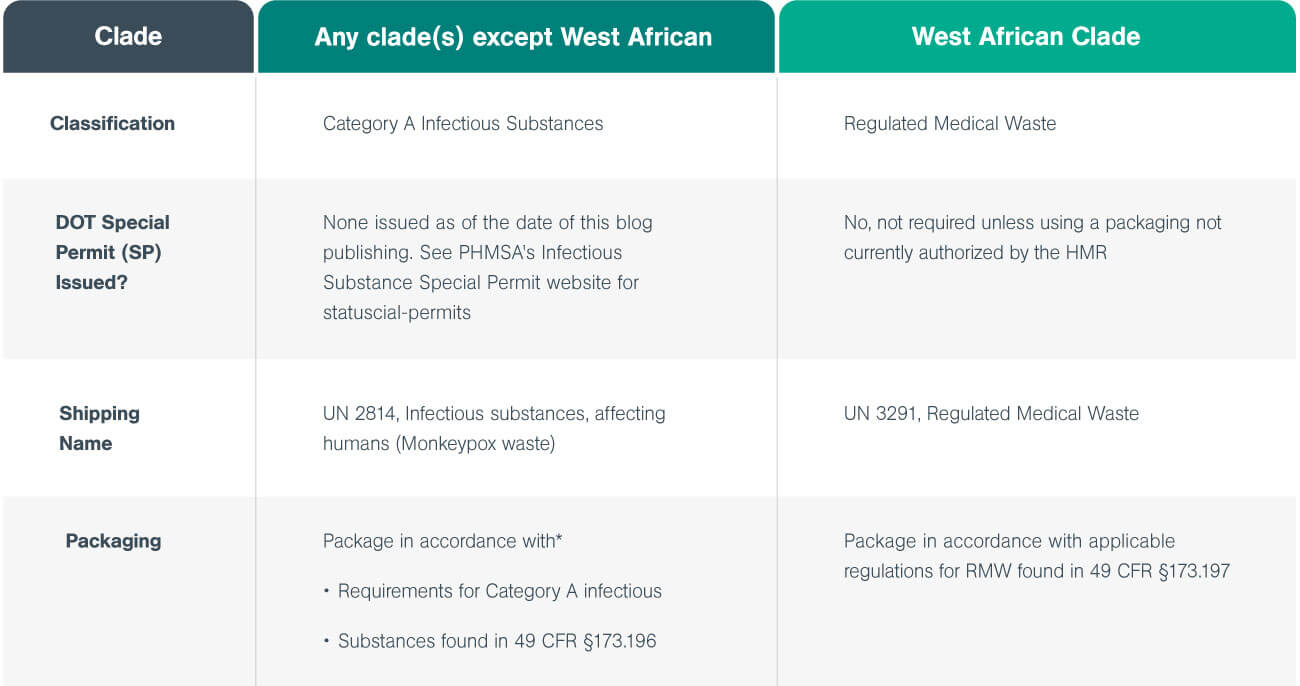
*PHMSA’s Infectious Substance Special Permit
West African clade wastes
As of publication of this document, per CDC Poxvirus Team:
“All isolates from the 2022 monkeypox outbreak have been characterized as West African Monkeypox. If the patient is thought to be a part of the current outbreak – say by an epidemiological factor – then there is a reasonable suspicion that it is west African clade. If the person had recent travel history to Dem. Repub. of Congo or Cameroon, then there could be a reasonable suspicion of Congo Basin. But, we have never found Congo basin in the US before. You do not need to wait for confirmation of clade.”
As of publication of this document, per DOT:
“If a clinician or their public health authority determine that a patient does not have known epidemiological risk for the Congo Basin clade of monkeypox, and recognizing that the current DOT guidance is to treat the West African clade as excepted from Category A, it is reasonable to manage waste from suspected monkeypox patients as UN3291 Regulated Medical Waste. However, if epidemiological risk factors indicate a risk for Congo Basin clade, waste should be managed as a suspected Category A infectious substance pending clade confirmation.”
Any clade except West African (Congo Basin)
Packaging and transport of Category A monkeypox waste is determined by USDOT and may require a special permit (SP). A USDOT SP would be required for alternative packaging designs not meeting HMR. Current packagings for Category A waste are almost exclusively designed to transport small samples between laboratories and are not adequate to move large volumes of waste. For example, in 2014, in order to potentially move large volumes of Ebola Waste, Daniels Health was issued a SP (16279) by the USDOT, authorizing alternative packaging, for Ebola only. Daniels is seeking this SP to accommodate Congo Basin clades should they occur in the US.
If you determine epidemiologically or via clade identification that a patient has Congo Basin clade, please contact your Daniels representative for further guidance on Category A packaging and transport requirements.
Turn to Expert Guidance for Help
Whenever dealing with a new or unexpected waste substance, it is important to ensure it is handled appropriately both to ensure the safety of all involved and to comply with the legal guidance from local, state, and federal authorities. Daniels Health has decades of experience dealing with waste from infectious diseases, so when in doubt, we are here to support your healthcare facility’s needs.
Let's Talk!
Your time is valuable, and we don’t want to play hard to get. You can either phone us directly on the details listed on our contact page, or feel free to fill out this short form and one of our team members will get back to you as quickly as possible.
